Respiratory syncytial virus (RSV) vaccination represents a significant advancement in preventive medicine, particularly for vulnerable populations including older adults and pregnant women. Unlike seasonal influenza vaccines that require annual administration, RSV vaccines follow a different dosing schedule that reflects the unique characteristics of immune response duration and disease patterns. Understanding the appropriate frequency of RSV vaccination is crucial for healthcare providers and patients alike, as it directly impacts protection against severe respiratory illness and hospitalisation.
The question of vaccination frequency becomes particularly relevant as healthcare systems worldwide implement RSV immunisation programmes. Current evidence suggests that RSV vaccines provide durable immunity lasting several years, fundamentally different from other respiratory virus vaccines. This distinction affects not only individual patient care decisions but also public health policy and resource allocation strategies across different age groups and risk categories.
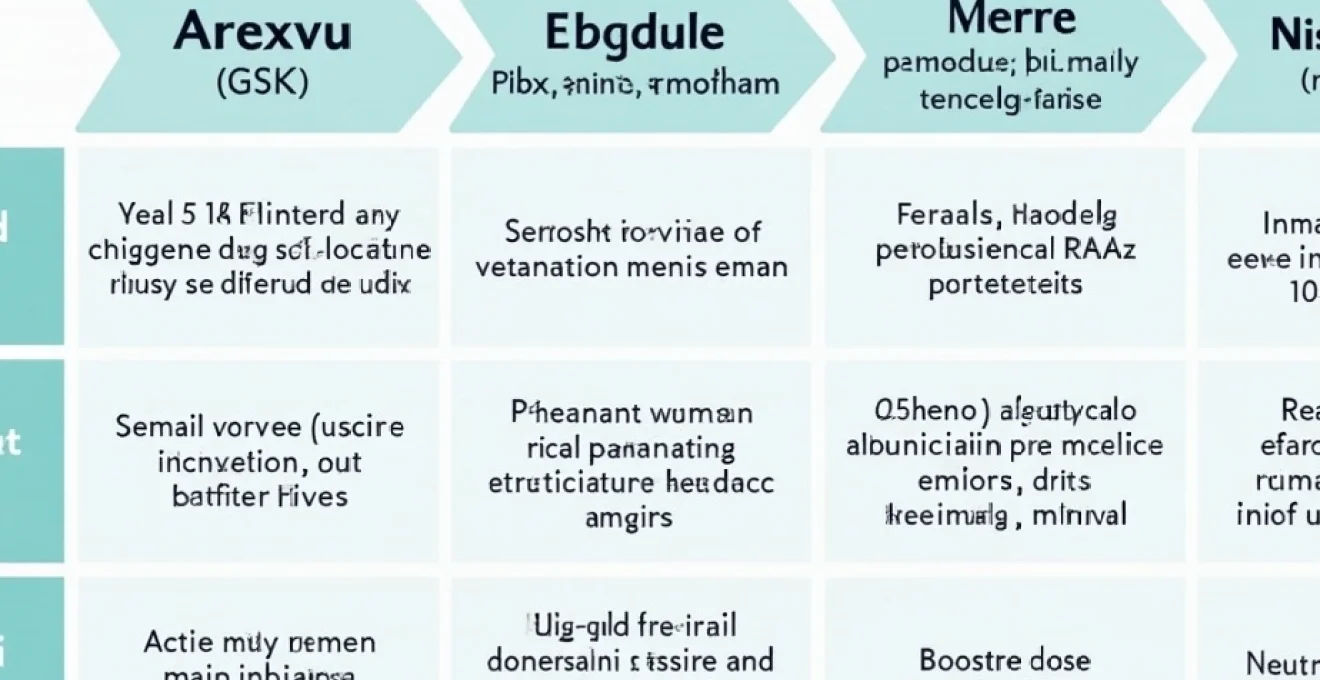
RSV vaccine types and formulations currently available
The landscape of RSV prevention includes several distinct vaccine technologies and prophylactic agents, each with specific dosing requirements and duration of protection. Understanding these different formulations helps clarify why vaccination frequency varies between products and patient populations.
Arexvy (GSK) adjuvanted recombinant protein vaccine
GSK’s Arexvy represents the first approved RSV vaccine for older adults, utilising a recombinant subunit approach combined with an adjuvant system. Clinical trials demonstrate that a single dose provides protection for at least two years, with effectiveness rates of approximately 77% against emergency department visits and 83% against hospitalisations during real-world use. The adjuvant component enhances immune response durability, contributing to extended protection periods that eliminate the need for annual revaccination.
Real-world effectiveness data from the 2023-2024 RSV season confirms sustained protection across diverse patient populations, including those with immunocompromising conditions and end-stage renal disease. The vaccine’s protein-based formulation triggers robust antibody responses that persist well beyond the initial vaccination period, making it a practical option for long-term RSV prevention strategies.
Abrysvo (pfizer) bivalent prefusion F protein vaccine
Pfizer’s Abrysvo utilises bivalent prefusion F protein technology, targeting both RSV A and RSV B subtypes with enhanced immunogenicity. This vaccine demonstrates similar durability to Arexvy, with single-dose protection lasting approximately 18 months in clinical trials and showing 79% effectiveness against emergency department encounters during real-world implementation. The bivalent approach provides broader coverage against circulating RSV strains, potentially extending protection duration.
Abrysvo’s unique formulation makes it suitable for both older adult vaccination and maternal immunisation programmes. When administered during pregnancy, maternal antibodies provide infant protection for approximately six months post-birth, demonstrating the vaccine’s versatility across different patient populations and protection strategies.
Mresvia (moderna) mRNA-1345 respiratory syncytial virus vaccine
Moderna’s mResvia employs messenger RNA technology similar to COVID-19 vaccines, instructing cells to produce RSV proteins that stimulate immune responses. Clinical trial data indicates approximately 80% efficacy during the first four months following vaccination, declining to 56% effectiveness at 12 months. This pattern suggests that mRNA-based RSV vaccines may require more frequent administration compared to protein-based alternatives.
The mRNA platform offers advantages in terms of manufacturing speed and adaptability to variant strains, though current evidence suggests potentially shorter protection duration. Post-marketing surveillance continues to evaluate long-term effectiveness patterns that will inform future dosing recommendations for this innovative vaccine technology.
Nirsevimab (beyfortus) monoclonal antibody prophylaxis
Nirsevimab represents a passive immunisation approach, providing immediate but temporary protection lasting approximately five months. Unlike vaccines that stimulate active immunity, this monoclonal antibody requires seasonal administration for continued protection. The product demonstrates 90% effectiveness in preventing infant hospitalisations when administered before RSV season onset.
This prophylactic agent fills a crucial gap for infants whose mothers did not receive maternal RSV vaccination, offering alternative protection strategies. The limited duration necessitates annual administration during peak RSV seasons, making it fundamentally different from longer-lasting vaccine approaches in terms of dosing frequency requirements.
Age-specific RSV vaccination schedules and intervals
Different age groups and clinical populations require tailored approaches to RSV vaccination frequency, reflecting varying immune system capabilities and disease risk profiles. These considerations directly influence optimal dosing intervals and revaccination strategies.
Adults aged 60 years and older primary vaccination timeline
For adults aged 60 and older, current recommendations support single-dose administration providing multi-year protection. The CDC recommends vaccination for all adults aged 75 and older, plus those aged 60-74 with increased risk factors including chronic cardiovascular disease, respiratory conditions, diabetes complications, and immunocompromise. Studies indicate protection lasting at least two years, with some evidence suggesting longer duration.
Primary vaccination timing optimally occurs during late summer or early autumn, before seasonal RSV circulation peaks. This strategic timing maximises protection during high-risk periods while accounting for the vaccine’s extended duration of immunity. Unlike influenza vaccination, annual RSV revaccination is not currently recommended for this population.
Pregnant women seasonal vaccination window (32-36 weeks gestation)
Maternal RSV vaccination follows a pregnancy-specific schedule rather than fixed calendar intervals. The vaccine should be administered during each pregnancy between 32-36 weeks gestation, preferably during September through January when RSV circulation is anticipated. This timing ensures optimal antibody transfer to the foetus while maximising infant protection during vulnerable early months.
Each pregnancy requires separate vaccination regardless of previous RSV immunisation history, as maternal antibody levels wane over time and each infant needs individual protection. The vaccination provides approximately six months of infant immunity, covering the period when babies face highest risks of severe RSV-related complications requiring hospitalisation.
High-risk adult populations accelerated dosing protocols
Adults with specific risk factors may benefit from modified vaccination approaches, though current guidelines do not establish different dosing intervals for high-risk populations. Risk factors include chronic heart or lung disease, end-stage renal disease, severe obesity, neurologic conditions affecting respiratory function, and moderate to severe immunocompromise. These populations demonstrate similar vaccine effectiveness to general adult populations in clinical studies.
Healthcare providers may consider individual patient factors when determining optimal vaccination timing, particularly for those with seasonal disease pattern variations or planned immunosuppressive treatments. However, standard single-dose recommendations currently apply across risk categories, with ongoing research evaluating whether certain populations might benefit from alternative dosing strategies.
Immunocompromised patients modified vaccination intervals
Immunocompromised adults may experience altered immune responses to RSV vaccination, though current evidence suggests similar protection duration compared to immunocompetent individuals. Studies include patients with various immunocompromising conditions, demonstrating vaccine effectiveness in preventing severe disease outcomes. However, individual immune response variations may influence optimal vaccination frequency.
Consultation with specialists managing underlying immunocompromising conditions helps optimise vaccination timing relative to treatment cycles and immune status fluctuations. While standard dosing intervals currently apply, future research may identify specific populations requiring modified vaccination schedules based on immune response monitoring and clinical outcomes data.
Clinical efficacy duration and waning immunity studies
Understanding how long RSV vaccines maintain protective immunity is fundamental to establishing appropriate revaccination intervals. Clinical trial data and real-world studies provide crucial insights into protection duration and potential waning patterns across different vaccine platforms.
Phase III trial data from RSVPreF3 OA OASIS study
The pivotal OASIS study evaluating Arexvy in older adults demonstrates sustained vaccine efficacy through multiple RSV seasons. Initial efficacy against RSV-associated acute respiratory illness reached 94% in adults aged 60-79 years, maintaining substantial protection levels throughout the study period. Follow-up data indicates continued protection against severe disease outcomes even as antibody titres naturally decline over time.
Long-term follow-up reveals that while antibody levels decrease from peak post-vaccination values, they remain well above pre-vaccination baselines for extended periods. Clinical protection against hospitalisation and severe respiratory tract disease persists beyond two years, supporting current single-dose recommendations for older adult populations.
Antibody titre persistence in MONeT trial participants
Moderna’s MONeT trial provides detailed immunogenicity data showing initial robust antibody responses followed by gradual decline over 12-18 months. Neutralising antibody titres remain elevated compared to baseline levels throughout the study period, though with notable decreases by month 12. This pattern influences current understanding of mRNA vaccine durability in RSV prevention.
The relationship between antibody levels and clinical protection remains under investigation, as some individuals maintain clinical protection despite lower measured antibody titres. These findings contribute to ongoing discussions about optimal revaccination timing for mRNA-based RSV vaccines compared to protein-based alternatives.
Real-world effectiveness studies from 2023-2024 RSV season
The first RSV season following vaccine licensure provides valuable real-world effectiveness data across diverse populations and healthcare settings. Studies demonstrate maintained protection against emergency department visits and hospitalisations, with effectiveness rates comparable to or exceeding clinical trial results. These findings support the durability of vaccine-induced protection in typical clinical practice environments.
Real-world effectiveness studies confirm that RSV vaccines provide substantial protection against severe disease outcomes during their first season of use, validating clinical trial predictions about protection duration.
Ongoing surveillance continues monitoring vaccine effectiveness patterns to identify any early signals of waning immunity that might influence future dosing recommendations. Current data supports existing guidance regarding vaccination frequency while building evidence for long-term programme optimization.
Neutralising antibody Half-Life against RSV A and B strains
Laboratory studies reveal distinct patterns in neutralising antibody persistence against different RSV strains, with implications for protection duration and potential revaccination needs. Antibodies against RSV A and RSV B subtypes demonstrate similar decay kinetics, with half-lives extending several months beyond initial peak responses. This balanced protection supports current vaccine formulations targeting both major RSV subtypes.
Understanding antibody kinetics helps predict clinical protection duration and informs decisions about booster dose timing. While laboratory measures provide important insights, correlation with clinical outcomes remains the primary determinant of vaccination interval recommendations in public health policy development.
Booster dose requirements and revaccination guidelines
Current evidence does not support routine booster dose administration for RSV vaccines in approved populations, distinguishing them from annual influenza vaccination strategies. The Advisory Committee on Immunization Practices (ACIP) and CDC continue evaluating whether specific populations might benefit from additional doses in the future, but present recommendations focus on single-dose primary vaccination series.
Several factors influence booster dose considerations, including individual immune response variations, emergence of variant strains, and duration of clinical protection in different populations. Ongoing surveillance studies monitor vaccine effectiveness over multiple RSV seasons to identify potential signals indicating need for revaccination strategies. Current guidance emphasises that individuals who received RSV vaccination in previous seasons should not receive additional doses at this time.
The approach to RSV vaccine boosters differs significantly from COVID-19 vaccination strategies, where frequent updates and boosters became necessary due to variant emergence and waning immunity patterns. RSV vaccines appear to provide more durable protection, though long-term follow-up continues evaluating whether periodic boosters might benefit specific high-risk populations or after extended time periods.
Unlike seasonal influenza vaccines, RSV vaccines currently provide multi-year protection that eliminates the need for annual revaccination in approved populations.
Future booster recommendations will depend on accumulating evidence from real-world effectiveness studies, immune correlate analyses, and potential emergence of RSV variants with reduced vaccine susceptibility. Healthcare providers should stay informed about evolving guidance as more data becomes available from extended post-marketing surveillance programmes.
Contraindications and special populations considerations
While RSV vaccines demonstrate excellent safety profiles across diverse populations, certain contraindications and special considerations affect vaccination frequency decisions. Individuals with severe allergic reactions to vaccine components should not receive RSV vaccines, requiring alternative protection strategies for those at high risk of severe disease.
Pregnancy status influences vaccination timing and frequency, with maternal vaccination recommended during each pregnancy rather than at fixed intervals. This approach ensures optimal antibody transfer to each infant while accounting for the specific gestational timing required for maximum effectiveness. Women who received RSV vaccination during one pregnancy should receive the vaccine again during subsequent pregnancies.
Special populations including immunocompromised individuals, those with autoimmune conditions, and patients receiving immunosuppressive treatments generally can receive RSV vaccines according to standard schedules. However, individual circumstances may warrant consultation with specialists to optimise vaccination timing relative to treatment cycles and disease management strategies.
Healthcare providers should consider individual patient factors when making vaccination frequency decisions, particularly for those with complex medical conditions or unique risk profiles. While standard recommendations apply to most eligible individuals, personalised approaches may benefit certain patients with specific clinical circumstances that affect immune response or disease risk patterns.
CDC and ACIP recommendations for RSV vaccination frequency
The Centers for Disease Control and Prevention maintains that RSV vaccination is not currently an annual vaccine, marking a significant departure from influenza vaccination programmes. Current recommendations specify single-dose administration for all adults aged 75 and older, plus adults aged 50-74 with increased risk factors for severe RSV disease. These guidelines reflect available evidence about protection duration and cost-effectiveness considerations.
ACIP continues reviewing accumulating data about vaccine effectiveness duration, immune response patterns, and potential need for revaccination strategies. Regular advisory committee meetings evaluate new evidence that might influence future recommendations about booster doses or modified vaccination intervals for specific populations. Current surveillance systems monitor vaccine effectiveness trends that will inform future policy decisions.
The recommendation framework emphasises risk-based vaccination for younger adults aged 50-74, focusing on those with chronic medical conditions that increase severe RSV disease risk. This targeted approach optimises resource utilisation while providing protection to those most likely to benefit from vaccination. Future expansions of recommendations may occur based on additional safety and effectiveness data.
International vaccination programmes provide additional insights into optimal RSV vaccination strategies, with similar single-dose approaches adopted in multiple countries. These parallel experiences contribute to global understanding of vaccine durability and appropriate dosing intervals across diverse populations and healthcare systems. Collaborative surveillance efforts enhance evidence quality supporting current frequency recommendations while identifying potential areas for programme refinement.